The importance of in vivo PDX modelling
Disease models are useful for the evaluation of therapeutic strategies, the elucidation of tumorigenic mechanisms and the identification of potential biomarkers. In vitro cultured cell lines and flank xenografting in immunocompromised mice have served as a reference for preclinical testing in cancer research for a long time. However, more recently, patient-derived xenografting (PDX) and ex vivo organ-like 3D (organoid) cultures are considered to be more clinically relevant (1).
In the past decades, much of the studies on cancer research have been conducted using in vitro assays on established human tumour-derived cell lines. Often these tumours were derived from relapsed metastatic lesions following intensive chemo and radiotherapy creating a bias towards the subclasses of your entity. Also, these cell lines are cultured on plastics in an artificial environment and adaptations have occurred during establishment which made them deviate from the original patient tumour. Further, the use of cell lines precludes insights into e.g. tumour heterogeneity, a major critical factor in tumour behaviour and drug response. Moreover, tumours will develop differently in a (human) body due to several tumour environmental elements, such as the presence of stroma or blood vessels. In order to achieve biological insights or monitor effects of drugging in an in vivo context, many studies used xenografts of these cell lines in immunocompromised mice or cell-line derived xenografting (CDX). The validity of these compound treatment experiments often has been questioned toward the clinical implications. The high failure rate (96%) in early clinical trials illustrates the urgent need for more predictive preclinical models as a major prerequisite for rapid bench-to-bedside translation of investigational anti-cancer therapies. Nevertheless, even up to today, it remains a common practice in the first steps of pre-clinical research. Alternatively, transgenic models can be used for translational studies but the possibility to work on different genetic backgrounds (e.g. mimicking chromosomal imbalances or additional mutations) is limited (2).
What is a PDX?
The development of so-called patient-derived xenografts (PDX) is offering exciting novel possibilities which, at least partially, overcome the above mentioned limitations. PDX models have been developed for several cancer entities, for which intact (i.e. not dissociated) (fresh) surgically-derived human tumour material or viable cells retrieved from a liquid biopsy such as blood or bone marrow sample is implanted into immunocompromised mice.
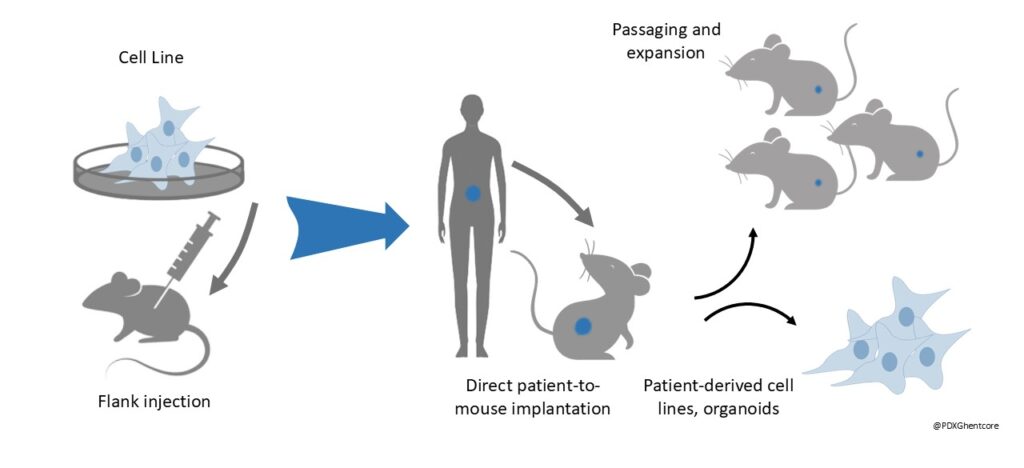
Figure 1: The transition from Cell-line derived xenografting (CDX) to Patient-derived xenografting (PDX)
PDXs have been shown to stably retain molecular, genetic and histopathological features of their originating tumours (1,3-4). As such, PDX models can most faithfully predict therapy response in distinct biological subtypes within a specific entity (3,5). Orthotopic PDX transplantation allows engraftment directly in the relevant organ therefore providing the best physiological cancer model available to date, including for the modelling of metastasis (1,4,5). Originally, their unique potential of being generated and studied in the laboratory in parallel with patient treatment in the clinic as avatars offers the opportunity for personalized medicine (1,4).
(1) Lum, D. H., Matsen, C., Welm, A. L. & Welm, B. E. Overview of human primary tumorgraft models: comparisons with traditional oncology preclinical models and the clinical relevance and utility of primary tumorgrafts in basic and translational oncology research. Curr. Protoc. Pharmacol. Chapter 14, Unit 14.22 (2012).
(2) Lieu, C.H., Tan, A.-C., Leong, S., diamond, J.R., Eckhardt, D.G. From Bench to Bedside: Lessons Learned in Translating Preclinical Studies in Cancer Drug Development. JNCI Journal of the National Cancer Institute. 105 (19), 1441-1456 (2013).
(3) Gengenbacher, N., Singhal, M. & Augustin, H. G. Preclinical mouse solid tumour models: status quo, challenges and perspectives. Nat. Rev. Cancer 17, 751–765 (2017).
(4) Gould, S. E., Junttila, M. R. & de Sauvage, F. J. Translational value of mouse models in oncology drug development. Nat. Med. 21, 431–439 (2015).
(5) Tentler, J. J. et al. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 9, 338–350 (2012).
Why a core facility?
There is an increasing demand for a more centralized approach that allows flexible exchange of models, protocols, and research tools to overcome several important disadvantages:
- By centralizing the acquirement of a sufficient number of immunodeficient mice, a more robust and efficient breeding can be achieved. The use of all offspring, regardless of age and sex, is ensured. This will significantly reduce the total number of animals used, and at the same time it guarantees the continuous availability of immunodeficient animals and decreases the risk of losing exclusive PDX models.
- The PDXGhent Core Facility, embedded within both UGent Core Facilities and UZ Gent, provides access to essential technology platforms and facilitates the use of patient materials and associated data. By bridging academia and clinical settings, it fosters collaborative translational research, driving innovation and advancing scientific discovery.
- To prevent that some of these samples and data get lost, a centralized approach is necessary that is managed by PDXGhent core and can be accessed by the different stakeholders. Long term backup storage will be done in the existing UZ Gent Biobank.
- Within the PDXGhent core, dedicated trained personnel focusses on generating models, performing in vivo drugging experiments, sharing standard operating procedures and up-to-date protocols; maintaining the database of models, and continued training to acquire new hands-on expertise.
The PDXGhent Core Facility is committed to centralized coordination, ensuring efficient and cost-effective workflows supported by dedicated, trained personnel, with a strong emphasis on the 3Rs. Offering both technical and administrative support, the facility aims to establish a comprehensive PDX biobank, intensify collaborations, and stimulate top-level cancer research.